Module on Thermal Equilibrium
The central alternative conception was that the students relate the equilibrium temperature of the objects, kept in a constant temperature enclosure, to the size and the material of the objects.
They do not necessarily think that this steady state temperature should be the temperature of surroundings. The central activity, therefore, was designed to show students how the temperature of objects, with different material and size, vary and finally reach the same steady state temperature equal to the temperature of the enclosure.
In the study, I found that the students did not understand the concept of thermal equilibrium itself.
I looked for possible analogy which could be demonstrated and such an experience could be harnessed to enable students for developing understanding of thermal equilibrium. The key point in understanding thermal equilibrium is the zero net heat (energy) flow across a boundary surface between two bodies. Since heat flow is rather difficult for the students to grasp as it is not directly amenable to perception, I considered hydrostatic equilibrium which is familiar to the students. The analogous net fluid (material) flow across an interface between two liquid systems can be demonstrated and is perceptible. Thus, the demonstration of the base domain (hydrostatic equilibrium), would make it easier for students to compare the sub-concepts of base domain and target domain (thermal equilibrium) and hence, help them extend their understanding to the target domain.
Further, the equilibrium depends on certain factors related to the process of exchange. Thus, such factors are important and when they are different, then the approach to the equilibrium may be different. This key aspect was demonstrated by developing the liquid flow analogy model for the central activity.
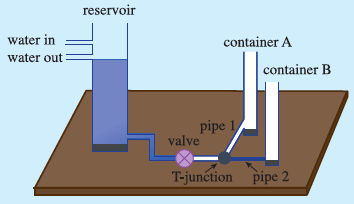
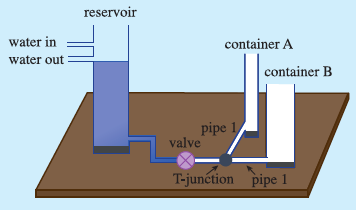
I had to take care that students didn’t take the analogy literally. I emphasised that in one case the quantity involved was matter whereas in the other case it was energy.